My son is trans-identified and his condition and the stress it has caused has torn my family apart. I will never stop advocating for him though, and doing my utmost to shine a light on the medical harms of the gender industry. To this end, I have spent countless hours reading and analyzing all available medical literature and research in this “field of medicine” (for lack of a better term) (see Part I and Part II). Lately, my focus has been on the link between autoimmune diseases and exogenous hormones and/or the blockade of hormones.
This is a topic close to home for me these days. Last year, my son commenced a self-prescribed and dangerous herbal concoction regimen. The dose levels he is taking are toxic. They contain high dose phytoestrogens and a herbal anti-androgen. Within months he started suffering from severe allergies ( 3 bouts of “allergies” lasting a total of 8 months) leading to significant stress and misery. His recent blood tests indicate alarming shifts in blood levels of Eosinophils, SHBG, monocytes and platelet counts that are risk factors for autoimmune disorder related allergy, liver damage, deep vein thrombosis, and CVD respectively. I decided to try to determine what is happening to his body and, at the same time, to look at other regimens prescribed for gender affirmation.
Here’s the state of research: We know that 80% of autoimmune disease sufferers are women and estrogen spikes are implicated significantly in their autoimmune disorders. At issue for these autoimmune diseases is the estrogen, not the “gender”—so, autoimmune disorders are also linked to both exogenous estrogen in males. Further, Testosterone deprivation in males is also linked to inflammation and chronic disease.
Despite the obvious risks, there is very limited research on the topic when it comes to gender medicine and it seems clear that patients enter the trans medical establishment with perfectly functioning endocrine systems and leave with induced endocrine disorders that are the supposed cure for their gender confusion—but yet exogenous estrogen is still being prescribed for gender care.
So, how is it possible that consistent, high dose exogenous estradiol given to boys as so-called gender affirmative treatment has escaped scientific research for safety and efficacy?
Simply put, the gender industry is choosing to not put these risks on their radar. And the gender industry is choosing not to conduct autoimmune tests, so they are not collecting any data that would support more research—because they know that these experiments would likely be difficult to justify if the research was there.
So, with the limited data we have, what do we know?
We know that we don’t know or understand the autoimmune effects.
In a recent research paper published on the topic of autoimmune effects of wrong sex hormones, it is clear that we know what we don’t know when it comes to autoimmune conditions and wrong sex hormones.
We know that there are likely autoimmune effects
The alarming immune and infection related research disclosures include several observations based on cause effect studies.
Sex hormones impact risk and pathology of autoimmune disease
Testosterone has been identified to upregulate AutoImmune REsponse (AIRE ) gene, whereas estrogen has been shown to decrease AIRE expression, reducing the efficiency of thymic self-tolerance and increasing incidence of autoimmune diseases.
Tregs are immune regulatory cells that are crucial for maintaining the balance between effective immune activation and self-tolerance. Decreased numbers of functional Tregs lead to failed self-tolerance and autoimmunity. The number of Tregs increases in the presence of testosterone in vitro. Increasing Estrogen and Reducing Testosterone is a double whammy for self tolerance and autoimmune disease activation in natal males!
Estrogen-based gender-affirming therapy may contribute to the development of SLE in genetically susceptible individuals. Anti-nuclear antibodies are considered a hallmark of SLE as they are detectable in over 97% of cases ( so called gender affirming care does not test for ANA or genetic susceptibility, WPATH SOC 8 recently released does not recommend any such testing!)
Estrogen or the reduction of androgens may increase the risk of Systemic Sclerosis development
The detection of autoimmune disease in a preclinical stage is important for preventing immune-mediated organ damage. Anti-nuclear antibodies may precede development of autoimmunity; a recent study observed that 31% of trans females were positive for anti-nuclear antibodies, compared with 13% in the male population. The trans male/female groups consistently reported higher positivity for anti-nuclear antibodies thereby increasing the risk for particularly those on estrogen treatment.
A hospital-based study investigated the incidence of MS in trans males and trans females, compared with cis males and cis females [Pakpour et al]. Between 1999 and 2011, this study included 1157 trans females and 2390 trans males; with 4.6 million cis males and 3.4 million cis females in the reference cohorts. From their results, trans females had an increased risk of developing MS with a risk ratio of 6.63 compared with females.
Testosterone may be protective in allergic disease, whereas oestrogen may aggravate allergic diseases, this is what I observed as I tested my son his allergies were so severe because he had knackered his free testosterone levels on a poisonous combination of high dose phytoestrogens and anti-androgenic plant based “supplements”.
In conclusion, given the immune-activating properties of estrogen, it seems likely that estrogen treatment may induce a lower threshold for immune activation in trans females. Therefore, natal males on estrogen and anti-androgens with a genetic susceptibility may be at an increased risk of developing disorders of immune hyperactivation, such as autoimmunity and allergy.
Epigenetic Cause Effect Study
A recent cause-effect epigenetic study at MCRI Australia is the first major study that systematically tracked changes in DNA methylation in people on wrong sex hormones. Their observations are significant and indicate how autoimmune diseases can be triggered by and sustained to organ damage by wrong sex hormones especially estrogens and anti-androgen “therapy” in healthy males. Their observations and results are startling:
Identified several thousand differentially methylated CpG sites (DMPs) (Δβ ≥ 0.02, unadjusted p value < 0.05) and several differentially methylated regions (DMRs) in both people undergoing feminizing and masculinizing GAHT, the vast majority of which were progressive changes over time.
Highlight the need to broaden the field of ‘sex-specific’ immunity beyond cisgender males and cisgender females, as transgender people on GAHT exhibit a unique molecular profile (as indicated above unknown and risky profile).
Gene promoters containing GAHT-associated DMPs are enriched for immune system processes, most markedly in genes that lose promoter DNA methylation during feminizing GAHT and genes that gain DNA methylation during masculinizing GAHT.
After 6 months and 12 months of feminizing GAHT, transgender women exhibit a significant loss of methylation of Vacuole membrane protein 1 (VMP1). In recent years, this region of VMP1 has been consistently reported as hypomethylated in blood of patients with inflammatory bowel disease (IBD), Crohn’s disease (CD), and ulcerative colitis (UC).
Systematic Reviews and Case Reports
In the seminal study “Sex Hormones in Acquired Immunity and Autoimmune Disease” on Estrogen on Immune system, V Moulton et al write:
Estrogens in general are considered immune stimulatory therefore pathogenic in autoimmune diseases. Systemic Lupus is a prototypical chronic systemic autoimmune disease and can affect any organ in the body. Joints and skin are frequently involved, while complications in vital organs such as kidneys can lead to lupus nephritis and renal failure. Complex interaction of genetics, environmental factors, and hormones lead to the deregulation and aberrant activation of the innate and adaptive immune systems leading to circulating autoantibodies and inflammatory immune cells which eventually lead to destruction of target organs . Historically, studies with gonadectomy/hormone deprivation and hormone supplementation in male and female lupus prone mice have shown a clear association of sex hormones with lupus, where estrogen accelerates or worsens disease and estrogen removal ameliorates disease in females. Male gonad removal increases susceptibility to disease in male mice and androgen supplementation improves disease in female mice. Human studies indicate that female hormones particularly estrogen plays important roles in immune cell generation, homeostasis, and function which impact control of immune responses. Estrogen mediates key effects on B cell physiology and function, which are vital in the pathogenesis of autoimmune diseases like SLE.. most important factors are concentrations and durations of Estrogen exposure.
The main auto immune diseases caused by wrong sex hormones in natal males that have been reported in several studies and anecdotally are: Crohn’s Disease or IBD, Multiple Sclerosis, Systemic Sclerosis, and Systemic Lupus Erythromatosis. These are severe, progressive and unfortunately fatal diseases.
In a study linking Estrogen with IBD, Pierdominici et al indicate that “Crohn disease (CD) and ulcerative colitis (UC) are chronic forms of inflammatory bowel disease (IBD) whose pathogenesis is only poorly understood. Estrogens have a complex role in inflammation and growing evidence suggests that these hormones may impact IBD pathogenesis”.
Studies of males with low T and high E levels.
In an alarming study of organ damage at high estrogen levels in males reported through the Medical University of South Carolina (MUSC) found that men with scleroderma and higher levels of estradiol had more severe disease and heart involvement. Those with the Scl-70 autoantibody and higher levels of estradiol had a greater risk of death. In scleroderma, the body makes too much connective tissue. This causes thickening of the skin and internal organs and, ultimately, organ damage. Women are three times more likely, and women in their child-bearing years nine times more likely to have the disease than men. Men, however, have more severe disease progression. Scleroderma develops in women during their child-bearing years, when estrogen levels are at their highest. Hormone replacement therapy trials found that women’s skin thickened during therapy and then returned to normal after completion of treatment. MUSC has previously reported that similar thickening occurred in skin cultures exposed to estradiol.
ANA markers and SCL-70 autoantibody tests are not administered in gender affirming “care” and any accelerating organ damage is likely not detected until it is too late.
The researchers in this study tested estradiol and scleroderma autoantibody levels in banked samples from 83 men aged 50 years and older with diffuse cutaneous systemic sclerosis, a type of scleroderma. They also tested samples from 37 healthy men of a similar age. They then used a variety of statistical approaches and the careful clinical annotations accompanying each sample to determine whether estradiol levels were linked to any of the clinical traits of scleroderma.
Male patients with diffuse cutaneous scleroderma had significantly higher levels of estradiol (average, 30.6 pg/mL) than both healthy men (average, 12.9 pg/mL) and postmenopausal women with the disease (24.2 pg/mL). Those with higher estradiol levels (average, 43.7 pg/mL) had significantly more heart involvement than those with lower levels (29.4 pg/mL). Finally, for patients with the Scl-70 autoantibody, increasing levels of estradiol in the serum was associated with a significantly greater risk of death.
People of African, Asian, and Native American ancestry are at higher risk for the disease than those of European descent. Those of African ancestry are also at greater risk for systemic sclerosis (SSc, scleroderma) and often develop SLE and SSc earlier in life and experience more severe disease than those of European descent ( self under siege article below).
Finally while the 2019 MUSC study found correlation to high estradiol in organ wall thickening in Systemic Sclerosis in vivo and in vitro cause effect studies, an earlier 2013 study made a direct link to circulating estrogen and organ wall thickening (Yasuoka et al). They concluded that the profibrotic effect of E2 and the increased circulating levels of E2 and estrone may explain, at least in part, the higher incidence of SSc.
Anecdotally, autoimmune effects are common and merit research
Understandably, the general public is not going to easily slog through the slim cause effect studies that exist. They are dense and technical. However, with the dramatic rise in transgender medicalization, we are rapidly amassing a host of anecdotal studies that, if known to the general public, would be much harder to ignore. Especially noteworthy are the stories are from detransitioners who, after medicalizing, report a host of ailments related to gender care.
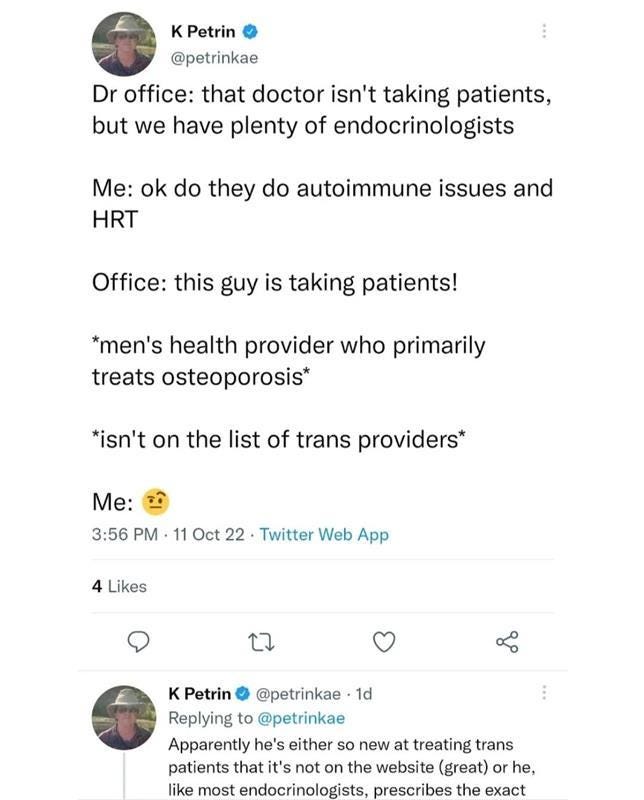
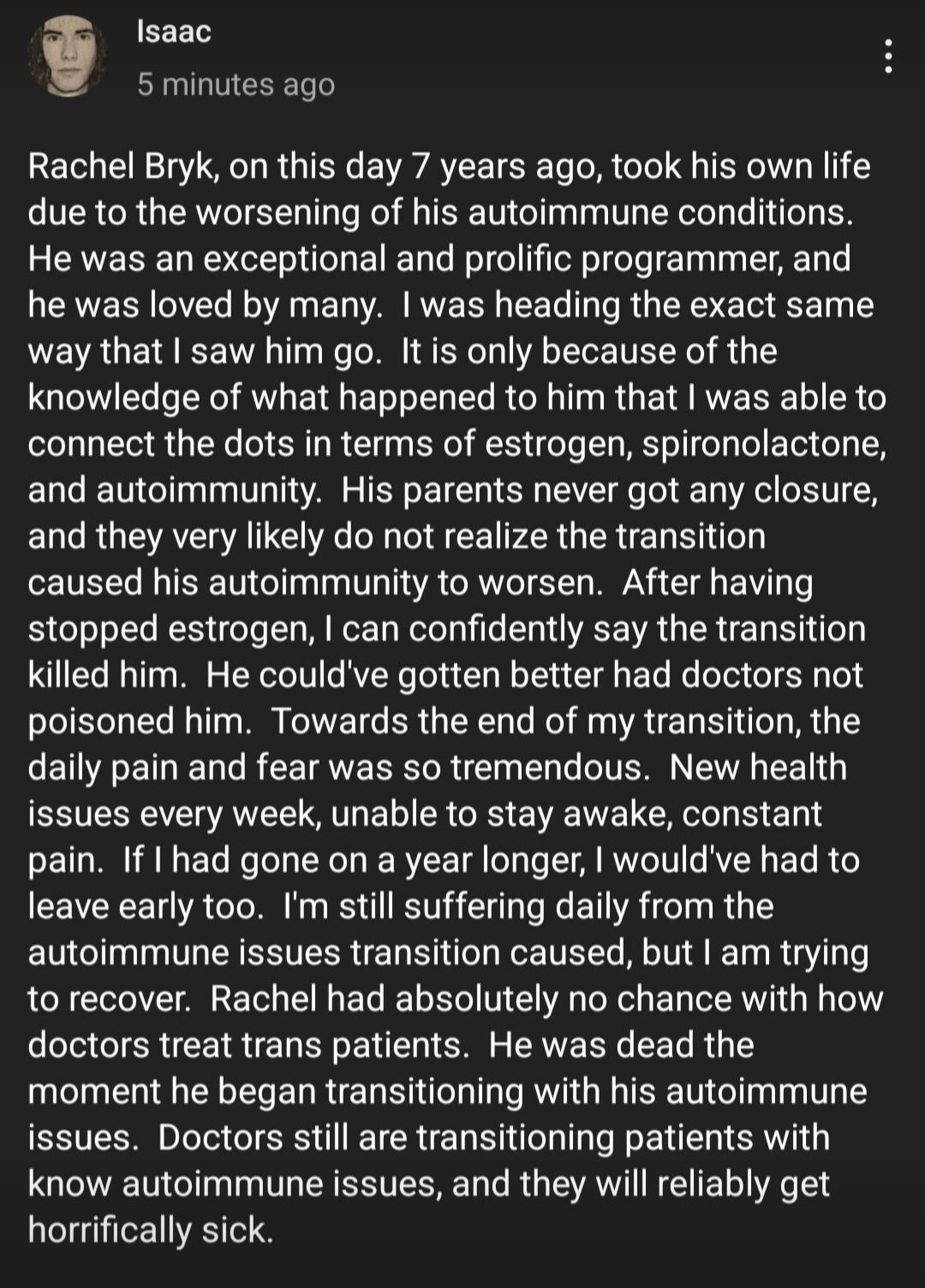
One such story is from Isaac, a brave detransitioner who has been writing about his autoimmune health-related issues that he attributes to his several years on Estrogen in the hopes that he can help spare others the effects of these wrong sex hormones. Isaac recently reported that his chronic health issues stemming from cross sex hormone use have essentially confined him to his home. He has discussed his health issues openly with the physician who prescribed him cross sex hormones without any testing for auto-immune sensitivity, and this physician blames Isaac for insisting on his own “treatment” path.
A random search on twitter of CSH and autoimmune disorders shows innumerable complaints by males on estrogen with many complaining of severe complications.
These anecdotes indicate that the medical community has failed many such individuals who have not been tested for genetic and autoimmune markers prior to initiation of wrong sex hormones.
It’s time for us to take a real look at the medical risks of wrong sex hormones, including the increased risk for autoimmune conditions. We need to acknowledge the many indications of risks, ban the experimental medications, and begin to collect data in a lab or controlled setting. It’s unconscionable to continue experimenting on humans for the sake of gender identity.
References
Aida-Yasuoka, K., Peoples, C., Yasuoka, H., Hershberger, P., Thiel, K., Cauley, J. A., Medsger, T. A., & Feghali-Bostwick, C. A. (2013, January 10). Estradiol promotes the development of a fibrotic phenotype and is increased in the serum of patients with systemic sclerosis - Arthritis Research & Therapy. BioMed Central. https://doi.org/10.1186/ar4140
Baker Frost, D., Wolf, B., Peoples, C. et al. Estradiol levels are elevated in older men with diffuse cutaneous SSc and are associated with decreased survival. Arthritis Res Ther 21, 85 (2019). https://doi.org/10.1186/s13075-019-1870-6.
Campochiaro C, Host LV, Ong VH, Denton CP. Development of systemic sclerosis in transgender females: a case series and review of the literature. Clin Exp Rheumatol. 2018 Jul-Aug;36 Suppl 113(4):50-52. Epub 2018 Feb 19. PMID: 29465362. https://pubmed.ncbi.nlm.nih.gov/29465362/
Dhindsa S, Champion C, Deol E, et al. Association of Male Hypogonadism With Risk of Hospitalization for COVID-19. JAMA Netw Open. 2022;5(9):e2229747. doi:10.1001/jamanetworkopen.2022.29747, https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2795874
Higher estrogen levels linked to more severe disease in scleroderma. (n.d.). Higher Estrogen Levels Linked to More Severe Disease in Scleroderma | MUSC | Charleston, SC. https://web.musc.edu/about/news-center/2019/06/05/estrogen-levels-scleroderma
Jasuja GK, de Groot A, Quinn EK, Ameli O, Hughto JMW, Dunbar M, Deutsch M, Streed CG Jr, Paasche-Orlow MK, Wolfe HL, Rose AJ. Beyond Gender Identity Disorder Diagnoses Codes: An Examination of Additional Methods to Identify Transgender Individuals in Administrative Databases. Med Care. 2020 Oct;58(10):903-911. doi: 10.1097/MLR.0000000000001362. PMID: 32925416; PMCID: PMC8010422. https://pubmed.ncbi.nlm.nih.gov/32925416/
Koenig, M. (2022, March 31). Raven Alexis dies at 35 after battling Crohn’s disease. Mail Online. https://www.dailymail.co.uk/news/article-10672379/Porn-star-Raven-Alexis-dies-35-battling-Crohns-disease-suffering-infection.html
Leffler J. Potential immunological effects of gender-affirming hormone therapy in transgender people - an unexplored area of research. Ther Adv Endocrinol Metab. 2022 Dec 10;13:20420188221139612. doi: 10.1177/20420188221139612. PMID: 36533187; PMCID: PMC9747891. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9747891/
Media, M. C. (n.d.). Gender-affirming hormone therapy can influence Gene Activity. Murdoch Children's Research Institute. Retrieved February 11, 2023, from https://www.mcri.edu.au/news-stories/gender-affirming-hormone-therapy-can-influence-gene-activity https://www.mcri.edu.au/news-stories/gender-affirming-hormone-therapy-can-influence-gene-activity
Moulton VR (2018) Sex Hormones in Acquired Immunity and Autoimmune Disease. Front. Immunol. 9:2279. doi: 10.3389/fimmu.2018.02279, https://www.frontiersin.org/articles/10.3389/fimmu.2018.02279/
Pakpoor J, Wotton, C, Schmierer K, Giovannoni G, Goldacre, M. Gender Identity Disorders and Multiple Sclerosis Risk: A National Record-Linkage Study (I5.008). Neurology Apr 2016, 86 (16 Supplement) I5.008; https://n.neurology.org/content/86/16_Supplement/I5.008
Pierdominici M, Maselli A, Varano B, Barbati C, Cesaro P, Spada C, Zullo A, Lorenzetti R, Rosati M, Rainaldi G, Limiti MR, Guidi L, Conti L, Gessani S. Linking estrogen receptor β expression with inflammatory bowel disease activity. Oncotarget. 2015 Dec 1;6(38):40443-51. doi: 10.18632/oncotarget.6217. PMID: 26497217; PMCID: PMC4747344. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4747344/
Schutte MH, Kleemann R, Nota NM, Wiepjes CM, Snabel JM, T'Sjoen G, Thijs A, den Heijer M. The effect of transdermal gender-affirming hormone therapy on markers of inflammation and hemostasis. PLoS One. 2022 Mar 15;17(3):e0261312. doi: 10.1371/journal.pone.0261312. PMID: 35290388; PMCID: PMC8923509. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8923509/
Self under siege. MUSC Health | Charleston SC. (n.d.). April 1, 2016, from https://muschealth.org/health-professionals/progressnotes/2016/spring/features/self-under-siege
Shepherd,Rebecca, Bretherton Ingrid, Pang Ken, Czajko Anna, Kim Bowon, Vlahos Amanda, Zajac Jeffrey D., Saffery Richard, Cheung Ada and Novakovic Boris. 'Gender Affirming Hormone Therapy induces specific DNA methylation changes in blood,' Clinical Epigenetics. DOI: 10.1186/s13148-022-01236-4
White AA, Lin A, Bickendorf X, Cavve BS, Moore JK, Siafarikas A, Strickland DH, Leffler J. Potential immunological effects of gender-affirming hormone therapy in transgender people - an unexplored area of research. Ther Adv Endocrinol Metab. 2022 Dec 10;13:20420188221139612. doi: 10.1177/20420188221139612. PMID: 36533187; PMCID: PMC9747891. https://pubmed.ncbi.nlm.nih.gov/29465362/



The information the author has so heroically amassed in this three-part series needs to be disseminated widely. PITT is a wonderful place to start, but it can't be the end of the line. How can this work receive the larger audience it deserves?
Very important information, but I cringe and rage when I see "cisgender" used in a medical format. Sex is a binary. There are males and females and a small, self-selected group of males and females who wish to inflict "transness" on themselves and the world. The rest of the world does not have to redefine itself to create the illusion that "trans" should be a significant, equal force.